HRA CLINICAL RESEARCH BOOT CAMP COURSE
COURSE REGISTRATION
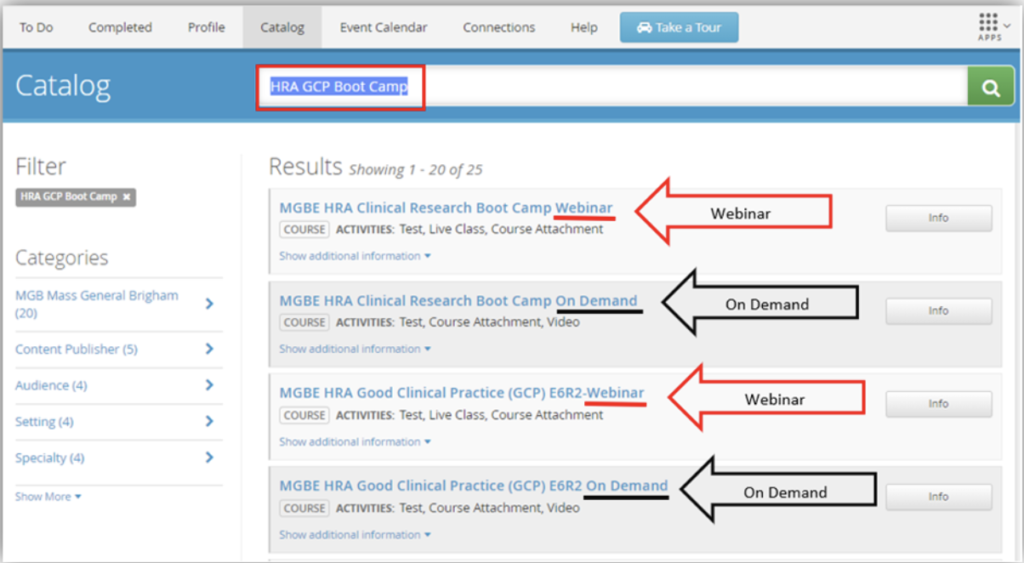
Registration for both the live webinar and the pre-recorded on-demand courses is available in HealthStream. Search for “HRA Clinical Research Boot Camp”
These courses are not assigned to members of the MGB research community. Users will need to register for the course in HealthStream. Click here for flipbook instructions for How To Register For On-Demand Courses in HealthStream.
COURSE DESCRIPTION
The Clinical Research Boot Camp course is a comprehensive human subjects research training course for investigators and all members of the study staff. Course content is based on the ethical principles of human research protections, Good Clinical Practice Guidelines, federal and state research regulations, institutional research policies, and reflects the 2018 Requirements of the Common Rule.
The principles established in the content of the course are applicable to all human research investigations. The content supports the protection of the rights, safety, and well-being of human participants and provides learners with the skills for applying the Belmont Report Principles and associated policies and regulations in common research practices.
COURSE FORMAT OPTIONS
- In-person, instructor led live webinar training – Eligible for RCR Credit!
- Pre-recorded course available on-demand
COURSE LENGTH
The course takes 2-2 ½ hours to complete.
*Recertification is required every 3 years from date of completion.
We do not manually update profiles in Insight. We receive course completion reports from HealthStream each weekday, and that report is uploaded to Insight each night, Monday-Friday. It takes two business days for the course completion information to be posted in Insight, which is the same as it was for CITI training.
