DEVELOP YOUR DMS PLAN
The new policy requires the submission of a Data Management and Sharing Plan (DMS Plan) that outlines how Scientific Data and any accompanying metadata will be managed and shared, taking into account any potential restrictions or limitations (see Mass General Brigham Policy below).
MGB LIMITATIONS AND RESTRICTIONS FOR DATA SHARING POLICY
The new NIH DMS Policy (effective January 25, 2023) promotes the sharing of scientific data and applies to all research that results in the generation of Scientific Data and is funded in whole or in part by NIH.
Under this policy, NIH recognizes that certain factors may limit the ability to preserve and share data. In response, Mass General Brigham released a systemwide policy that describes limitations and restrictions for sharing data when creating a Data Management Plan (DMS Plan) as required by the NIH DMS Policy. The Mass General Brigham policy includes:
- Legal and contractual limitations/restrictions for sharing data
- Technical limitations to sharing data
- Human data: ethical and legal limitations/restrictions on sharing data
- Animal data: ethical and legal limitations/restrictions on sharing data
Please be sure to familiarize yourself with this policy when writing your next DMS Plan (see MGB Template for DMS Plans for Mass General Brigham-specific guidance). A quick reference summary of the policy can be found here.
6 NIH DMS PLAN COMPONENTS
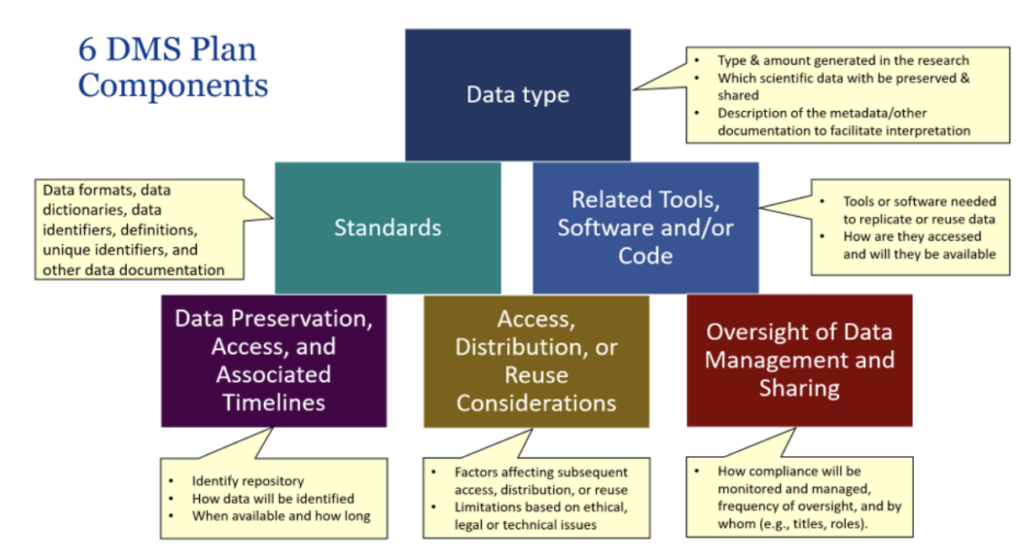
CHOOSING A REPOSITORY
Researchers should ensure that any repository they choose meets the characteristics and considerations as described in the NIH Desirable Characteristics for All Data Repositories as well as the Additional Considerations for Repositories Storing Human Data.
Researchers should follow the process outlined in the diagram below and information that follows for identifying the appropriate repository or other method for sharing data and metadata. This information should be described in Section 4 of the DMS Plan “Data Preservation, Access, and Associated Timelines.”
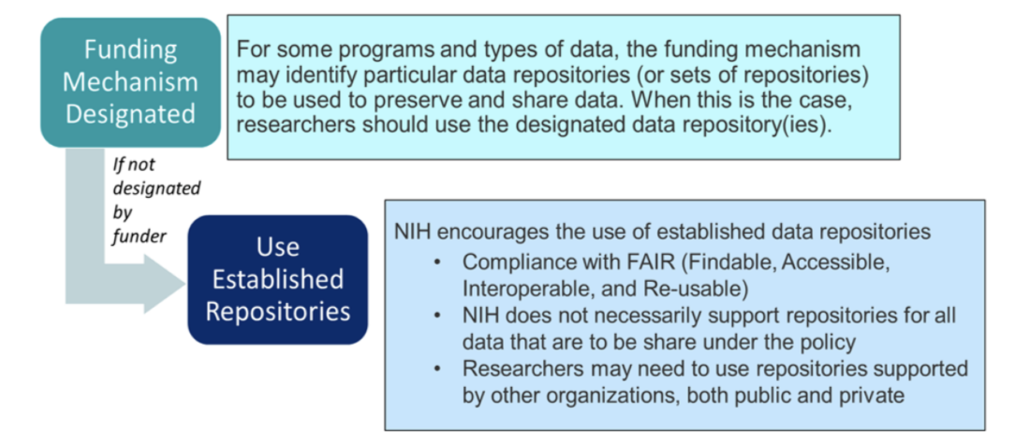
If no appropriate discipline or data-type specific repository is available, researchers should consider a variety of other potentially suitable data sharing options
- Small datasets (up to 2 GB in size) may be included as supplementary material to accompany articles submitted to PubMed Central
- Data repositories, including generalist repositories in compliance with FAIR (Findable, Accessible, Interoperable, and Re-usable), or institutional repositories, that make data available to the larger research community, institutions, or the broader public.
- Large datasets may benefit from cloud-based data repositories for data access, preservation, and sharing.
ACCEPTED/RECOMMENDED REPOSITORIES
Repositories supported by the NIH Common Fund, NCATS, NCCIH/ODS, NCI, NEI, NHGRI, NICHD, NCI, NIHGRI/NIGMS, NHLBI, NIA, NIAAA, NIAID, NIBIB, NIMH, NIDA, NICHD, NIDCD. *Note IC’s may change.
Generalist repositories accept data regardless of data type, format, content, or disciplinary focus. NIH does not recommend a specific generalist repository and provides a non-exhaustive list as a guide for locating generalist repositories. A comparison chart can help you determine which generalist repository fits your needs.
The Harvard Dataverse Repository is a free data repository open to all researchers from any discipline, both inside and outside of the Harvard community, where you can share, archive, cite, access, and explore research data. Each individual Dataverse collection is a customizable collection of datasets (or virtual repository) for organizing, managing, and showcasing datasets.
Vivli is an independent, non-profit organization that has developed a global data-sharing and analytics platform. The Vivli platform includes an independent data repository, in depth search engine and a secure research environment. Users can search listed studies, request data sets from data contributors, aggregate data or share data of their own. The secure research environment includes access to robust analytical tools.
REQUEST USE OF OTHER REPOSITORIES
In rare or unusual circumstances in which your data are not eligible or appropriate for one of the repositories noted above and listed below, researchers must obtain approval for utilization of other external repositories. To request approval:
- Complete the repository checklist to verify your chosen repository meets the NIH-recommended characteristics
- Send an email to: mgbarchitecturecouncil@partners.org
- Put in the subject line that your request is research-related. Describe what repository or other means you are proposing to use to share your data and metadata and provide a justification for why the NIH or other accepted/recommended generalist repositories do not meet your needs
- Requests must be sent at least two weeks in advance of a grant deadline to ensure adequate time for review
