CLINICAL RESEARCH BILLING
Clinical research billing compliance is the process of ensuring that all clinical services related to a research study are billed appropriately and in accordance with all applicable federal, state, and third-party regulations; all study-related materials, including the sponsored agreement, informed consent form, and study budget; and all MGB policies and procedures. Accurate clinical trial billing is the responsibility of the PI.
Clinical research billing processes are often complex as costs associated with research-related clinical services can be incurred at multiple Mass General Brigham organizations over the course of a trial. In addition, clinical research often takes place in conjunction with routine care visits for patients in trials, making it essential that billing for each service is appropriate and accurate. The convergence of research and clinical care reimbursement in clinical research trials necessitates regulations to prevent improper billing of federal health care programs and violating the Federal False Claims Act, the penalties of which are severe. The regulations stipulate
- Do not bill for services that have already been paid by the sponsor (double billing)
- Do not bill for services that are required for research purposes only
- Do not bill for services that were promised free to the subject in the informed consent form
CLINICAL RESEARCH BILLING PROCESS
For any CMS qualifying trial, the Mass General Brigham Clinical Trial Office (CTO) is responsible for conducting a Medicare Coverage Analysis (MCA) to determine eligibility for coverage of study-related items or services. Routine Costs are determined through a review of the protocol and standard treatment guidelines in consultation with the Principal Investigator and study staff. The CTO creates a billing grid that mirrors the protocol schedule of events and includes notations of what should be billed to the research sponsor and what may be billed to Medicare. This billing grid becomes the guide for appropriately directing clinical research charges. Research charge allocation occurs in Epic and is performed by the study team. Study team members tasked with performing research charge allocation MUST complete Clinical Research Billing Compliance training.
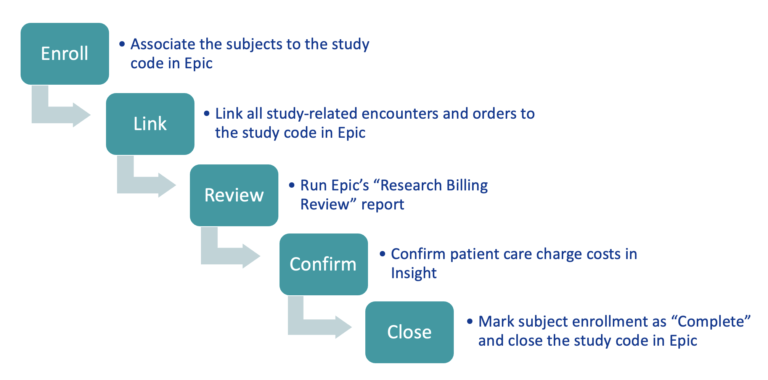
The charge allocation process consists of 5 essential steps outlined below